GEM Allograft Bone Matrices
We have used our extensive orthopedic experience to find regenerative products that we believe best meet, and exceed, the needs of the surgical dentist. A proprietary process delivers greater surface area to facilitate revascularization and support in-growth of new bone.
Why is GEM ALLOGRAFT unique?
- Every lot tested and verified osteoinductive, in contrast to most other bone allograft
- Our line of osteoinductive fibers and putties are extensively tested utilizing an animal implant model and in-vitro AP model to confirm inductive potential
- Higher surface area generated by the elongated particulate shapes, not the “rocks” produced by cheaper (and more expensive) alternatives
- Higher surface area facilitates greater stimulation of revascularization and in-growth of new bone
- All products achieve sterility assurance levels (SAL) of 10-6, and are processed in accordance with AATB Standards and FDA regulations
Lynch Biologics’ GEM Allografts are PROCESSed using:
- All products are processed aseptically using proprietary BioRinse™ decontamination technology, and in accordance with AATB Standards and FDA federal regulations.
- BioRinse™ is validated decontamination process that uses proprietary rinsing agents in multiple combinations designed to kill pathogenic microorganisms, vegetative bacteria and spores. These steps include the removal of debris, blood, bone marrow and lipids, and components associated with disease transmission.
- BioRinse™ in combination with a final low-dose sterilization step under low-temperatures ensures a sterility assurance level (SAL) of 10-6 in accordance with a Sterile “R” claim, while preserving the osteoinductive potential of the graft.
Allograft tissue processing partner facts
- All DBM products verified for Osteoinductivity; Every lot, Post-Sterilization. Both In-Vivo & In-Vitro testing performed
- All products have a Sterility Assurance Level (SAL) of 10-6
- United States (U.S.) Food & Drug Administration (FDA) Registered
- American Association of Tissue Banks (AATB) Accredited
- Company only processes donated human tissue
- Donors come from U.S. and Canada Registered Recovery Agencies
- To date, no company product has been recalled or withdrawn
GEM ALLOGRAFT BONE MATRICES
MINERALIZED Bone Fibers
- GEM Cortical FDBA: Mineralized Cortical Particulate allograft provides all the benefits of cortical bone, including greater amounts of BMPs compared to cancellous bone, and is a slower resorbing material, ideal for space maintenance and increased mineralized tissue for longer times
- GEM Cancellous FDBA: Mineralized Cancellous Particulate provides increased surface area and porosity which allows for capillary ingrowth, which in turn promotes formation of osteoblasts for new bone growth
- GEM Cortical Cancellous FDBA: Mineralized Cortical/Cancellous Particulate (approximately 60/40 mix) blends the advantages of cortical and cancellous FDBA.
- Osteoconductive
- Sterile – Sterility Assurance Level (SAL) or10-6 Five-year shelf life
- 100% allograft bone – no additional carrier
- Room temperature storage
Particulate Cortical Grafts – Small Particle 0.25 – 1.0mm
- .25cc | LBFDBASP025
- .5cc | LBFDBASP05
- 1.0cc | LBFDBASP1
- 2.0cc | LBFDBASP2
Particulate Cancellous Grafts – Small Particle 0.25 – 1.0mm
- .25cc | LBCFDBA025
- .5cc | LBCFDBA05
- 1.0cc | LBCFDBA1
- 2.0cc | LBCFDBA2
Particulate Cortical Cancellous Grafts – Small Particle 0.25 – 1.0mm
- .25cc | LBCCFDBA025
- .5cc | LBCCFDBA05
- 1.0cc | LBCCFDBA1
- 2.0cc | LBCCFDBA2
Particulate Cortical Cancellous Grafts – Large Particle 1.00 – 2.0mm
- 1.0cc | LBCCFDBAL1
- 2.0cc | LBCCFDBAL2

DEMINERALIZED BONE matrices – VERIFIED OSTEOINDUCTIVE
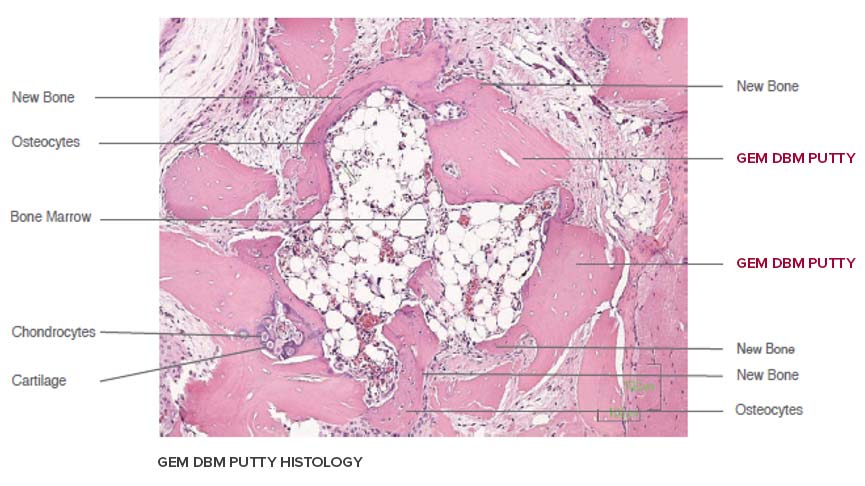
Each lot of Lynch Biologics GEM Demineralized Bone Matrix that is incorporated in the Fibers and Putties below, has histologically demonstrated the presence of all five (5) elements of bone formation including new bone, bone marrow, osteocytes, chondrocytes and cartilage in the athymic rat post-implantation model at 28 days. In vivo testing is performed by an independent laboratory on every lot of GEM DEMINERALIZED BONE MATRIX product POST STERILIZATION to ensure VERIFIED OSTEOINDUCTION.
These products have been processed to preserve native BMP’s and provide for an unparalleled osteoactive graft.
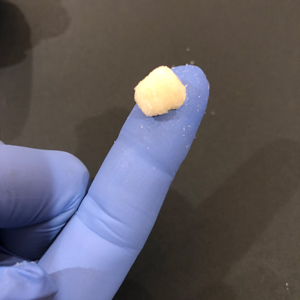
Moldable ALLOMIMETIC Putty
MOLDABLE ALLOMIMETIC PUTTY is THE solution for those indications where migration of a graft is not acceptable, and you require a verified osteoinductive material. The proprietary combination of our particulates and fibers with NO inert carrier to block capillary ingrowth and cellular migration. Migration resistant while sustaining the desired hydrating constituent.
MOLDABLE ALLOMIMETIC PUTTY is verified for osteoinductivity, each lot, post-sterilization prior to release for distribution. In-vivo test results have histologically demonstrated the presence of all 5 bone-forming elements (chondrocytes, osteocytes, bone marrow, cartilage, and new bone).
MOLDABLE ALLOMIMETIC PUTTY does not contain any extrinsic carriers and is made from 100% human bone.
MOLDABLE ALLOMIMETIC PUTTY is provided sterile with a sterility assurance level (SAL) 10-6. The product should be stored in room temperatures (59-86° F or 15-30° C) and has a shelf-life of up to 5 years from the date of packaging.


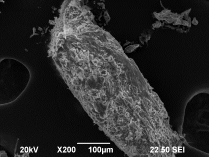
DEMINERALIZED CORTICAL Bone FIBERS
DEMINERALIZED CORTICAL BONE FIBERS have been processed utilizing a proprietary process and have histologically demonstrate the presence of all five (5) elements of bone formation including new bone, bone marrow, osteocytes, chondrocytes and cartilage in the athymic rat post-implantation model at 28 days. In-vivo testing is performed by an independent laboratory on every lot of GEM DEMINERALIZED BONE MATRIX product POST STERILIZATION.
DEMINERALIZED CORTICAL BONE FIBERS may be hydrated with saline, sterile water, blood, or protein containing solutions in accordance with clinicians well-informed medical judgment. GEM DEMINERALIZED CORTICAL FIBERS do not contain extrinsic carriers, and are derived entirely from 100% human allograft bone. GEM DEMINERALIZED CORTICAL FIBERS are provided in a ready-to-use mixing jar.
DEMINERALIZED CORTICAL BONE FIBERS are provided sterile with a Sterility Assurance Level (SAL) 10-6. The product should be stored at ambient temperatures and has a shelf life of five-years from date of packaging.
- Osteoinductive and osteoconductive
- 100% demineralized cortical fibers
- Sterile – Sterility Assurance Level (SAL) or 10-6
- Five year shelf life
- BioRinse™ process removes cellular components associated with inflammatory response while leaving matrix intact.
- Ambient storage temperatures (dental sizes)
- 1.0 cc | LBDCF1
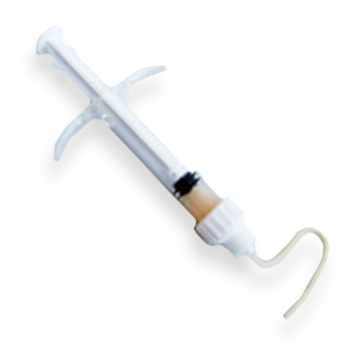
Demineralized Flowable Putty in Syringe
DEMINERALIZED FLOWABLE PUTTY IN SYRINGE is derived of 100% allograft bone and processed using a proprietary demineralization process.
DEMINERALIZED FLOWABLE PUTTY IN SYRINGE has histologically demonstrated, post sterilization, to exhibit all five (5) elements of bone formation.
DEMINERALIZED FLOWABLE PUTTY IN SYRINGE does not contain any extrinsic or inert carriers.
DEMINERALIZED FLOWABLE PUTTY IN SYRINGE is provided in a ready to use syringe.
- Osteoconductive and Osteoinductive
- 100% allograft bone – no additional carrier
- Stays where you put it; Resists compression from the flap, and displacement from irrigation
- Sterile – Sterility Assurance Level (SAL) or 10-6
- Two-year shelf life
- Room temperature storage
- Available in delivery syringe
- 0.5 cc | LBDCM05
- 1.0 cc | LBDBM1